CD Laboratory for Molecular Stress Research in Peritoneal Dialysis
Head of research unit
Commercial Partner
Duration
01.03.2023 - 29.02.2024
Thematic Cluster

The body reacts to cell-damaging substances with a cytoprotective molecular stress response. The aim of this work is to better understand and positively influence the underlying mechanisms using peritoneal dialysis as a model.
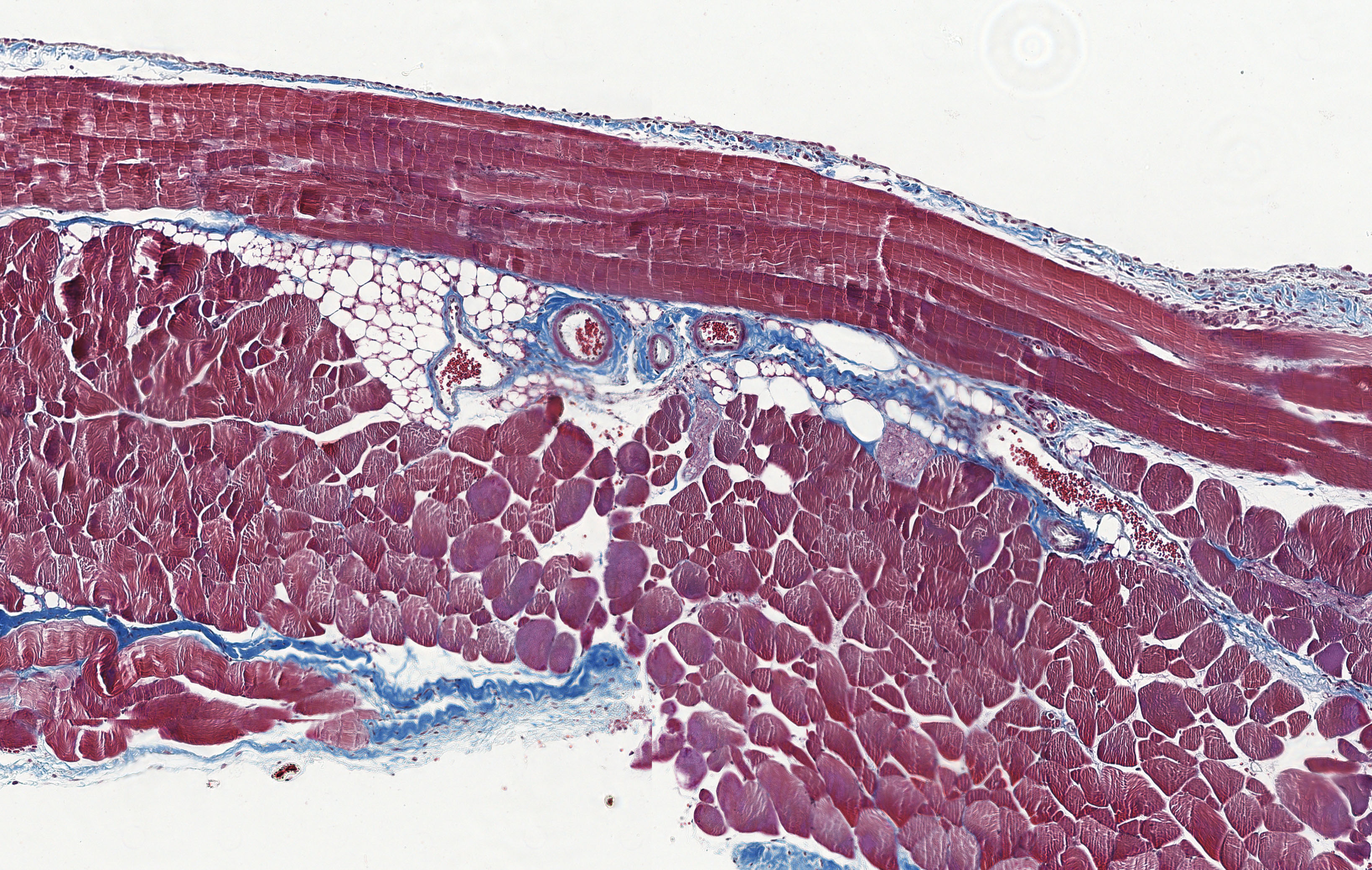
Peritoneal dialysis is a form of dialysis in which a dialysis fluid is introduced into the abdominal cavity via a catheter. The peritoneum is used as a dialysis membrane, and substances that are excreted via the urine in healthy people reach the abdominal cavity and are drained via the catheter.
Studies by the research group have shown that the dialysis fluids used in peritoneal dialysis not only damage the mesothelial cells of the peritoneum, but that a cytoprotective stress response is triggered as a protective mechanism immediately after contact with this stressor. This is proportional to the toxicity and therefore the bioincompatibility of the peritoneal dialysis fluid.
In a molecular stress response, routine processes within the cells are reorganised in order to adequately counter the stressor. For example, so-called chaperones, e.g. heat shock proteins (HSP), are produced. These are proteins that help other proteins to maintain their folding and thus their position in the event of stress caused by toxins. If necessary, chaperones can also modify other proteins so that they can fulfil different positions as required.
However, the cell stress caused by peritoneal dialysis does not occur in this form in nature and evolution has not prepared the human body to react adequately. As a result, the cellular stress response is inadequate and misdirected processes occur. The new proteins created as part of the stress response send the wrong signals in PD patients. The aim of this research project is therefore to investigate how the peritoneal dialysis fluid can be modified so that the cellular mechanisms are supported and function properly again.
Peritoneal dialysis, which regularly and predictably induces the same cell damage in peritoneal mesothelial cells, is an optimal model to study the mechanisms of the cellular stress response and to test cytoprotective interventions based on heat shock proteins.
Glutamine has shown such a cytoprotective effect in earlier studies and its efficacy and tolerability have also been well studied in the context of tube and intravenous feeding of patients requiring intensive care. It is already used clinically in its more stable form as alanyl-glutamine. As an additive to peritoneal dialysis fluid, alanyl glutamine is intended to support the body's molecular stress response. In order to understand the effects of added alanyl-glutamine, this CD Laboratory will identify new biomarkers for peritoneal dialysis fluid-induced deleterious mechanisms and validate their predictive value for clinical use.
Secondly, the molecular mechanisms by which the dialysis fluid has a cytoprotective effect are being investigated. In a first step, this research approach focuses on the role of O-GlcNcylation in restoring the adequate cellular stress response of peritoneal cells, the target proteins and signalling pathways involved. Further potential mechanisms of action are the subject of future investigations.
Peritoneal dialysis, as home dialysis performed independently by the patient, enables patients, including those who live far away from a dialysis centre, to receive adequate renal replacement therapy, which is, however, subject to systemic toxicity and cannot be continued without restriction due to an imbalance between the degree of cell damage and the onset of the cellular stress response. The research of this CD Laboratory aims to elucidate the mechanisms of this stress response and find ways to support it and thus enable long-term tolerable home dialysis.
Christian Doppler Forschungsgesellschaft
Boltzmanngasse 20/1/3 | 1090 Wien | Tel: +43 1 5042205 | Fax: +43 1 5042205-20 | office@cdg.ac.at

