CD Laboratory for Production of next-level biopharmaceuticals in E.coli
Head of research unit
Commercial Partner
Duration
01.01.2024 - 30.04.2024
Thematic Cluster

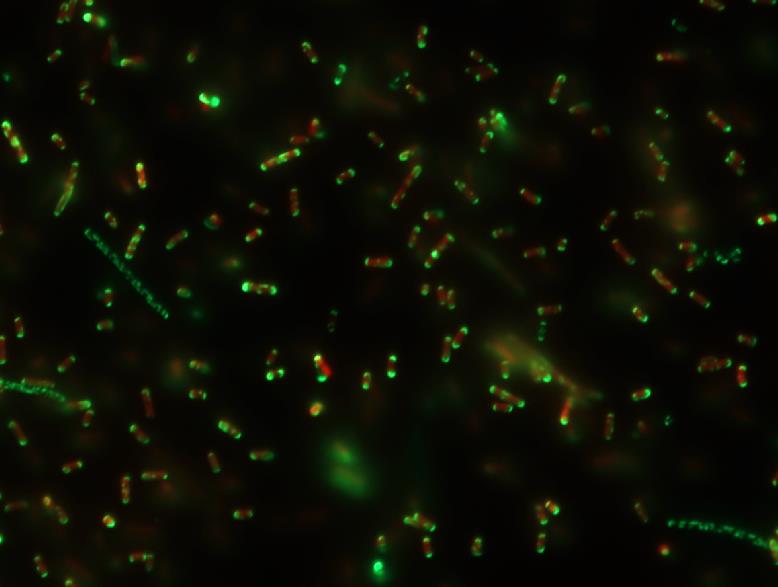
Biopharmaceuticals are drugs, mostly recombinant proteins, which are produced in living cells. This CD Laboratory investigates the scientific basis for the efficient production of novel proteins with increased efficacy in E. coli.
Next-generation biopharmaceutical active ingredients are mostly biotechnological, i.e. proteins produced with the help of bacteria or animal cells, which are equipped with novel functionalities and specific binding properties. On the one hand, this makes them highly effective drugs with few side effects, but their production is extremely difficult due to their often very complex structures and binding properties. In this CD Laboratory, the scientific basis and solutions for the efficient production of these proteins will be developed using E. coli bacteria. As this bacterial production system is part of the human intestinal flora, it is frequently used for the biotechnological synthesis of drugs.
The production of synthetic, bioactive proteins takes place in three steps. In the first step (cell engineering), the E. coli bacteria are genetically modified in such a way that they produce (express) proteins with the desired properties. The second step (fermentation) is the large-scale production of the proteins in bioreactors. Finally, the production batch must be purified and the desired protein isolated. There are interdependencies between all these process steps, which will be investigated on a basic scientific basis in this CD Laboratory. Process engineering aspects will be placed at the centre of the work and special attention will be paid to the interdisciplinary interfaces between cell engineering, fermentation and product purification.
Antibody fragments will serve as representative model proteins for this research work. These are regarded as a promising, cost-effective and very efficient alternative to current immunotherapy. Antibody fragments only contain the specific antigen binding site. New expression systems are also being used. These allow the investigation of long-term system stability under production conditions. It will also be investigated to what extent changes in the conditions during fermentation and variations in the expression system have an influence on the efficiency of the subsequent process steps and thus on the product quality.
In the field of protein processing, a novel system based on microparticles will be studied. The basic principles of this processing method must be researched on a broad basis and the method must be further developed for the production of novel biopharmaceuticals.
The approach of investigating application-related issues on a broad basic scientific basis and developing solutions from this will make a significant contribution to the production of novel, difficult-to-produce proteins.
Christian Doppler Forschungsgesellschaft
Boltzmanngasse 20/1/3 | 1090 Wien | Tel: +43 1 5042205 | Fax: +43 1 5042205-20 | office@cdg.ac.at

