CD Laboratory for Advanced Crystal Engineering Strategies in Drug Development

This CD Laboratory aims to research critical material properties of pharmaceutical excipients and active pharmaceutical ingredients that are crucial for the production, quality and safety of high-quality drugs. Optimising these properties makes it possible to accelerate manufacturing processes and ultimately reduce the costs of essential drug products.
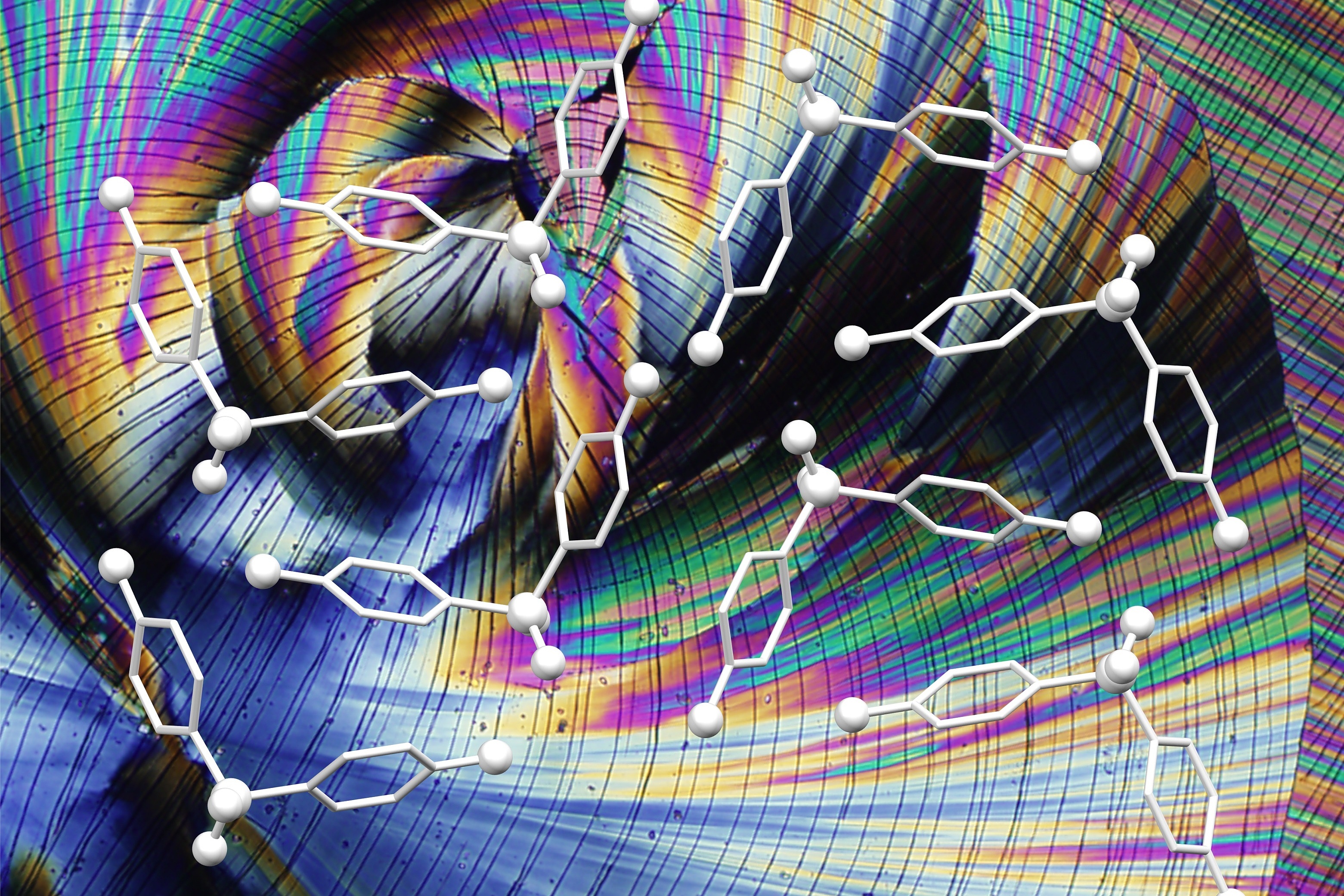
The vast majority of currently available active pharmaceutical ingredients consist of small molecules, which, for reasons of chemical stability, are usually processed in a crystalline state into solid dosage forms such as tablets. However, the structural diversity and, above all, the complexity of drug substances has increased enormously in the last two decades, which inevitably results in a deterioration of critical solid-state properties, such as water solubility.
Targeted optimisation strategies for drug substances are therefore increasingly the focus of modern drug development. Such efforts are largely based on material science approaches such as ‘crystal engineering’. Dozens of crystal forms can be produced from most drugs using sophisticated strategies and methods, and their properties can differ significantly. The challenge is to identify the form(s) with the best properties in terms of physical and chemical stability, solubility, processability, mechanical properties, etc. This is a fundamental prerequisite for the further development of a biologically active substance into a marketable drug.
The design and synthesis of solid structures with the desired properties requires a thorough understanding of the principles of molecular interaction and a broad research methodology. The topic encompasses a wide range of solid-state phenomena and the spectrum of solid forms ranges from crystals consisting of only one type of molecule with different packing arrangements (polymorphism) to complex multi-component crystals (co-crystals) and non-crystalline (amorphous) solids.
The overarching goal of this CD Laboratory is to explore and establish an understanding of the interplay between molecular features and supramolecular properties that influence the chemical and physical stability, processability, and biological efficacy of drugs. This is achieved by combining a variety of computational and experimental methods and processes.
In addition to optimising the material properties of new active ingredients, research also includes innovative, formulation-related topics. The CD Laboratory focuses on a comprehensive understanding of the formation of solid forms, their stability and production-relevant properties. This enables better predictability of the solid forms. Directives for customised experimental and virtual methods for finding new solid phases, as well as new strategies for stabilising metastable solid forms, are also being developed. This requires a sound knowledge of the molecular interactions between the active pharmaceutical ingredient and the excipients (co-formers), which are obtained from experimental or computer-generated structures. This also allows the prediction of process-relevant properties of drugs, which are essential for drug manufacturing processes.
Christian Doppler Forschungsgesellschaft
Boltzmanngasse 20/1/3 | 1090 Wien | Tel: +43 1 5042205 | Fax: +43 1 5042205-20 | office@cdg.ac.at

